Individuals diagnosed with mild to intermediate macular degeneration might benefit from genetic testing to assess their risk of progressing to the more severe and vision-threatening advanced form of the condition.
Patients often ask, “My mother/father had macular degeneration. Is it hereditary? Will I develop it too? What steps should I take?” These are crucial inquiries. So, let’s delve into our current understanding of the genetics behind this condition.
Here’s a breakdown of the macular degenerative process: The macula, situated at the central part of our retina, contains the highest concentration of light-sensitive neuro-receptors. This area demands the most energy, oxygen, and nutrients in the retina due to its specialized cells. Consequently, it is highly susceptible to factors that reduce these essential supplies, such as cardiovascular disease, diabetes, and poor nutrition. Lifestyle choices like diet and exposure to environmental toxins can also impact blood flow, oxygen levels, and nutrient delivery. A compromised macula is particularly vulnerable to environmental factors like UV light and smoking.
Here’s a closer look at the progression;
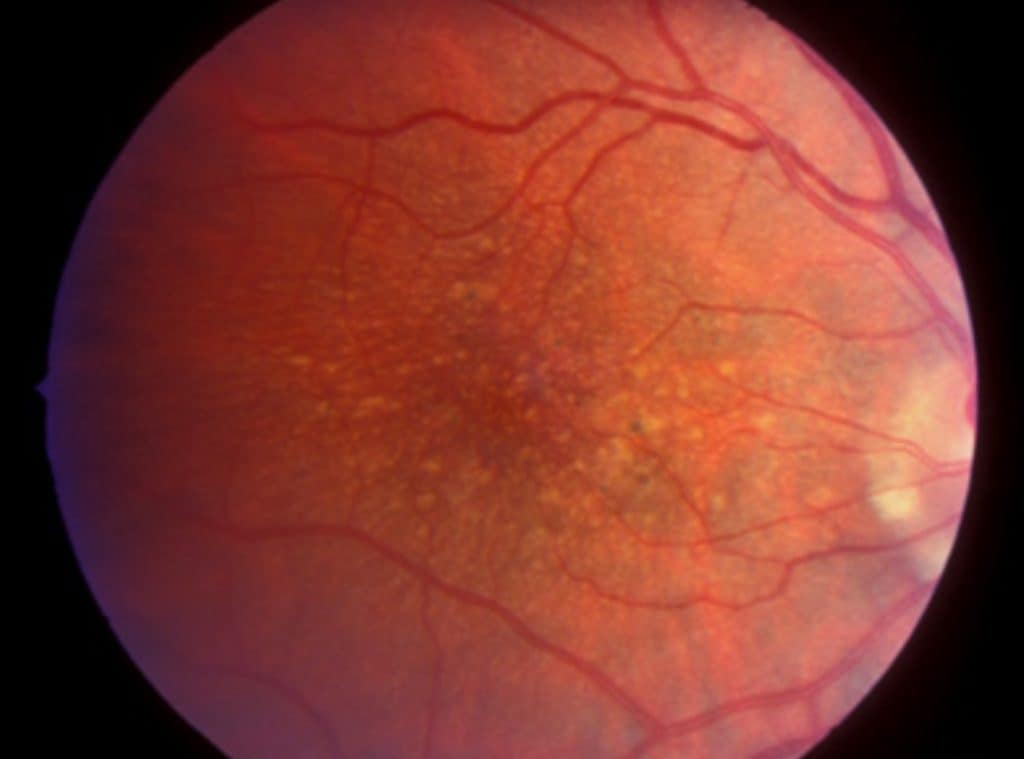
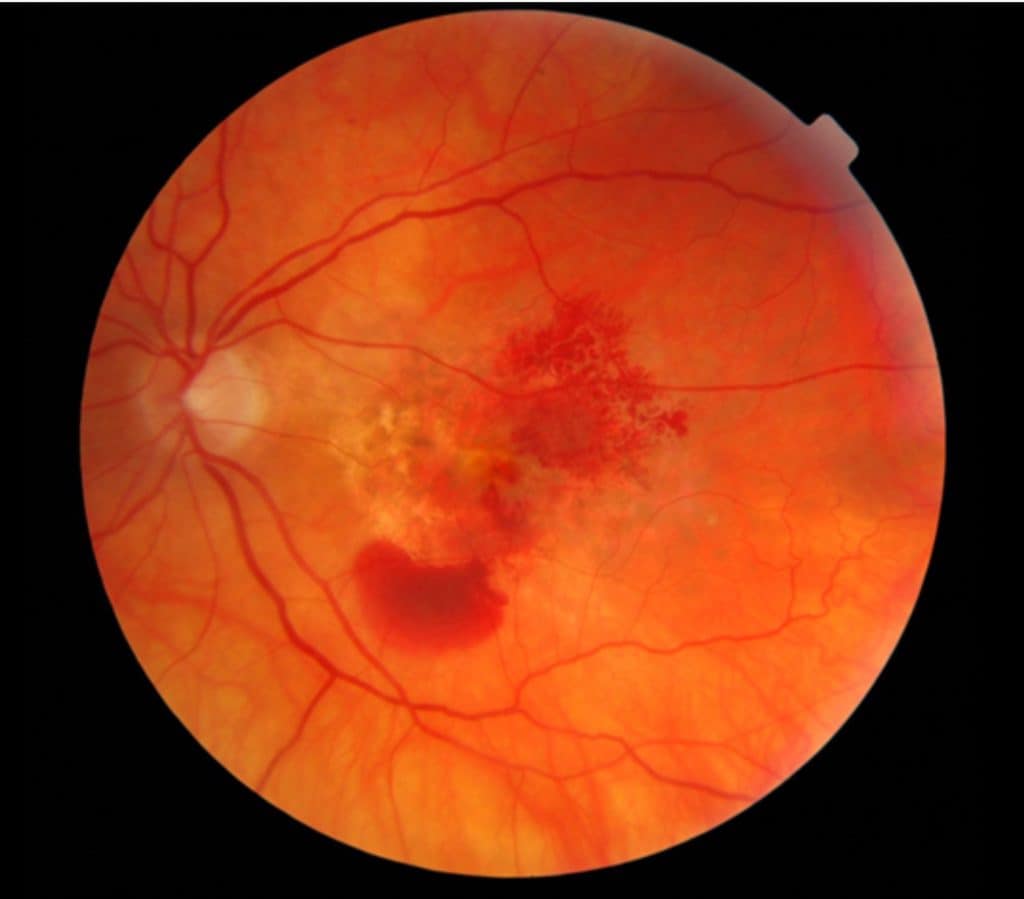
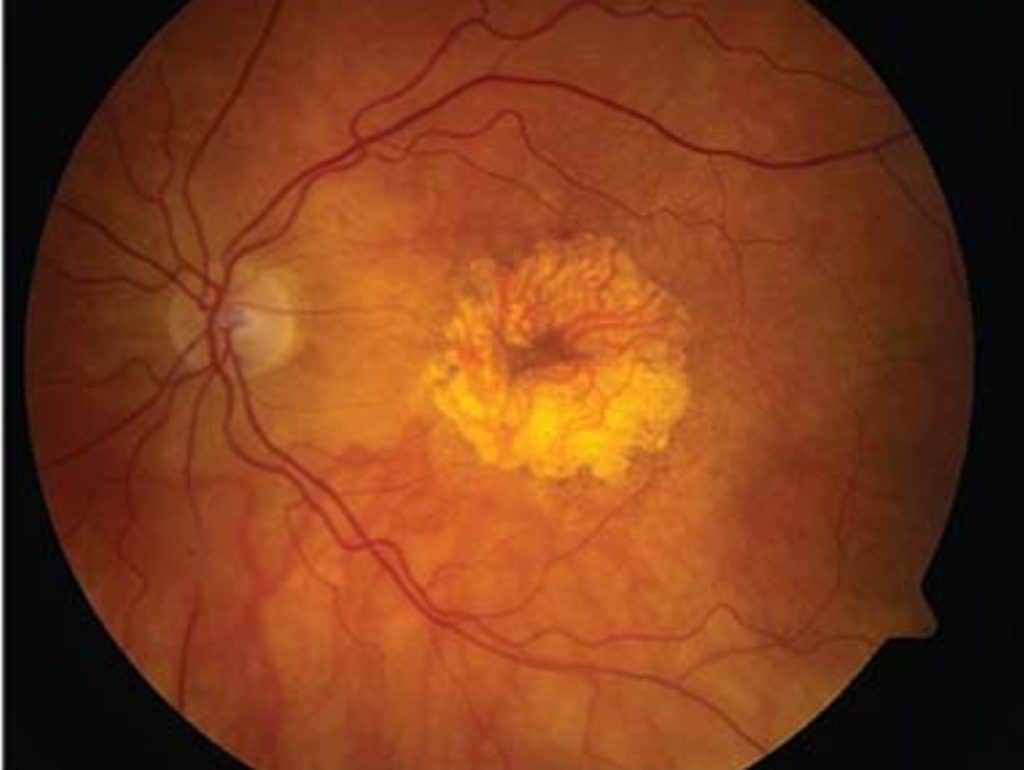
So it is known that general health and environmental factors affect our risk for developing macular degeneration. Factors that are somewhat controllable. But the question is…..
Does genetics play a role, and how much of a role does it play?
Studies have revealed that there isn’t a single ‘macular degeneration gene’; rather, age-related macular degeneration (AMD) is a multifaceted disease with various genetic factors at play. Instead of a single gene, there are specific areas (gene sequences) on the chromosome that influence the structure and function of the macula. This leads to different phenotypes among individuals, where different genes interact in diverse ways, influenced by both genetics and the environment. It might sound complex, but this complexity underscores the challenges in developing genetic tests to assess AMD risk.
Numerous studies have focused on AMD and its genetic associations. Two gene variants, in particular, stand out as significant contributors to the degenerative process: the complement factor H (CFH) gene on chromosome 1 and the age-related maculopathy susceptibility 2/HtrA serine peptidase (ARMS2/HTRA1) genes on chromosome 10. These are just a couple of the many gene variants that could potentially contribute to AMD risk. However, the significance of CFH and ARMS2/HTRA1 lies in the fact that multiple studies have consistently confirmed their role in AMD. Individuals carrying these genes are at an increased risk of developing advanced AMD, particularly when exposed to certain environmental factors.
An analogy often used is that having these genetic variants is akin to having a time bomb within us as we age. Aging itself is considered a significant risk factor for macular degeneration, hence the term Age-Related Macular Degeneration (AMD).
What Genetic Tests are Available ?
Two genetic tests currently available for macular degeneration are the Macula Test PGX and Vita Test, both by ArticDx. These tests analyze genetic markers on known genes associated with AMD.
Vita Risk (ArticDx)
This test specifically examines genetic markers associated with AMD. Notably, a significant finding revealed that a particular genetic profile caused AMD progression in some individuals using the AREDS supplement formula, particularly due to its zinc content. Genetic testing serves as a personalized guide to determine the most suitable supplements for reducing the risk of disease progression. Armed with this knowledge, eye doctors can advise patients against taking the AREDS formula with zinc, showcasing the concept of personalized medicine. Not all individuals benefit equally from the AREDS vitamin supplement due to varying genetic profiles.
Macula Risk (ArticDx)
Developed to predict AMD risk and severity, this test utilizes known genetic components of macular degeneration. It is intended for those with early or intermediate AMD. Research indicates that different genes and gene loci play distinct roles in various stages of AMD progression. For instance, the CFH gene is linked to inflammation, while the ARMS2/HTRA1 gene is associated with neovascularization and ‘wet’ macular degeneration.
click to connect to ArticDx website
How is the Test Conducted?
The test is a simple in-office procedure that your eye care professional must order. The doctor swabs the inside of the cheek, and the sample is sent to ArticDx for analysis. Results are typically available in about 4 weeks.
The doctor receives the report indicating the patient’s risk category, ranging from Category 1 to Category 5. The lab assesses the genetic testing results of 12 genes combined with non-genetic factors (such as smoking) to determine the risk category. Individuals in Categories 4 and 5 are at the highest risk for advanced AMD.
ArticDx’s research data indicates an 89.5% accuracy in predicting progression over 10 years and 88.3% accuracy over 5 years.
What’s the Purpose of Macular Risk Testing?
The aim of Macular Risk testing is to predict which individuals with early and intermediate AMD are likely to progress to the advanced stage. With knowledge of this genetic predisposition, you and your doctor can implement dietary and lifestyle changes and establish a monitoring schedule.
While routine genetic testing isn’t currently standard practice, it is an available option. Without genetic testing, managing treatment and controlling health factors are effective strategies to slow AMD progression. Note that these genetic tests may not be covered by all insurance plans; however, Medicare began reimbursing for genetic testing for AMD in July 2017.
The hope is that a deeper understanding of the genetics of eye diseases will lead to new preventative and treatment therapies in the future.
Potential Role of Gene Therapy in Macular Degeneration
Gene therapy for macular degeneration is currently in the research phase. The concept behind gene therapy is to replace or remove defective genes in the cells of the targeted tissue, particularly in the case of AMD affecting the retinal tissue. The process begins with identifying the affected cells and pinpointing the defective gene.
Researchers then develop a replacement gene. The challenge lies in the delivery method: how to transport the replacement gene to the targeted cells. This requires a vector, usually a virus, which naturally infects cells. However, in gene therapy, the virus’s native genetic material is replaced with the engineered replacement gene, eliminating concerns about viral infection.
While this sounds straightforward, gene therapy has been in development for decades and is a highly intricate process. For a more detailed explanation of gene therapy, you can visit [Genetics Home Reference, Medline ](link here).
Currently, researchers are focusing on macular diseases such as inherited retinal dystrophies like Stargardt’s Disease, Best’s Disease, Sorsby’s Disease, and cone dystrophies. These diseases share a commonality in that their gene defects are identified and relatively well understood. They fall under the category of monogenic diseases, meaning they involve a single defective gene.
However, the disease process and causes of AMD are not fully comprehended at this time. The specific role of genes is not entirely clear, but certain genes mentioned earlier in this discussion predispose individuals to AMD. Due to the multifactorial nature of macular disease genetics, it is unlikely, at least for now, that gene therapy will be a viable option.
In the End…
Genetic testing for macular degeneration can provide valuable insights into an individual’s risk of progression to advanced stages of the disease.
While gene therapy remains in the research phase and is not yet a viable option for AMD, understanding the genetics of macular diseases like Stargardt’s Disease and Best’s Disease sheds light on potential treatment directions.
Ultimately, whether to pursue genetic testing is a decision best made in consultation with your eye care professional. While it may not be standard practice, it offers a proactive approach to managing AMD risk factors. As research continues, we hope for advancements leading to new preventative and treatment strategies in the future.
Want to learn more about macular degeneration? Read: The 7 Truths about Macular Degeneration